The Maladaptive Notch Reactivation in Obesity-Induced Glucose Homeostasis
My post-doctoral training with Domenico Accili raised several interesting questions, specifically, the novel role of Notch in glucose metabolism. Notch is a highly conserved family of proteins critical for cell fate decision-making, but prior to our studies, less was known about Notch action in mature tissue. We showed that Notch signaling is present at low levels in normal physiologic conditions, but increases markedly in livers from diet-induced or genetic mouse models of obesity. To test the repercussions of this increased Notch signal, we generated mouse models lacking hepatocyte Notch signaling. These mice, when challenged with high-fat diet feeding, showed improved glucose tolerance due to reduced FoxO1 action on gluconeogenic promoters (Pajvani et al, Nature Medicine, 2011; Ozcan et al, Cell Metabolism, 2012). In euglycemic-hyperinsulinemic clamp studies in obese mice, we found that pharmacologic Notch inhibitor treatment improves hepatic insulin sensitivity with little effect on glucose disposal, confirmed by parallel studies in adipocyte-specific Notch loss-of-function mice (Sparling et al, Molecular Metabolism, 2015). These studies also revealed the unexpected role of Notch signaling to regulate β cell maturity and proliferation (Bartolome et al, Journal of Clinical Investigation, 2018); in sum, these data provide the groundwork to repurpose these agents to address the dual pathologies that characterize type 2 diabetes – insulin resistance and β cell dysfunction (Pajvani and Accili, Diabetologia, 2015).
Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld AL, Shulman GI, Kitajewski J, Accili D. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nature Medicine, 2011. DOI: 10.1038/nm.2378 | PubMed
Sparling DP, Yu J, Kim K, Zhu C, Brachs S, Birkenfeld AL, Pajvani UB. Adipocyte-specific blockade of gamma-secretase, but not inhibition of Notch activity, reduces adipose insulin sensitivity. Molecular Metabolism, 2015. DOI: 10.1016/j.molmet.2015.11.006 | PubMed
Pajvani UB, Accili D. The new biology of diabetes. Diabetologia, 2015. DOI: 10.1007/s00125-015-3722-5 | PubMed
Bartolome A, Zhu C, Sussel L, Pajvani UB. Notch signaling dynamically regulates adult β cell proliferation and maturity. J Clin Invest., 2019. DOI: 10.1172/JCI98098 | PubMed
Notch as a Causal Factor for NAFLD/NASH and Dyslipidemia
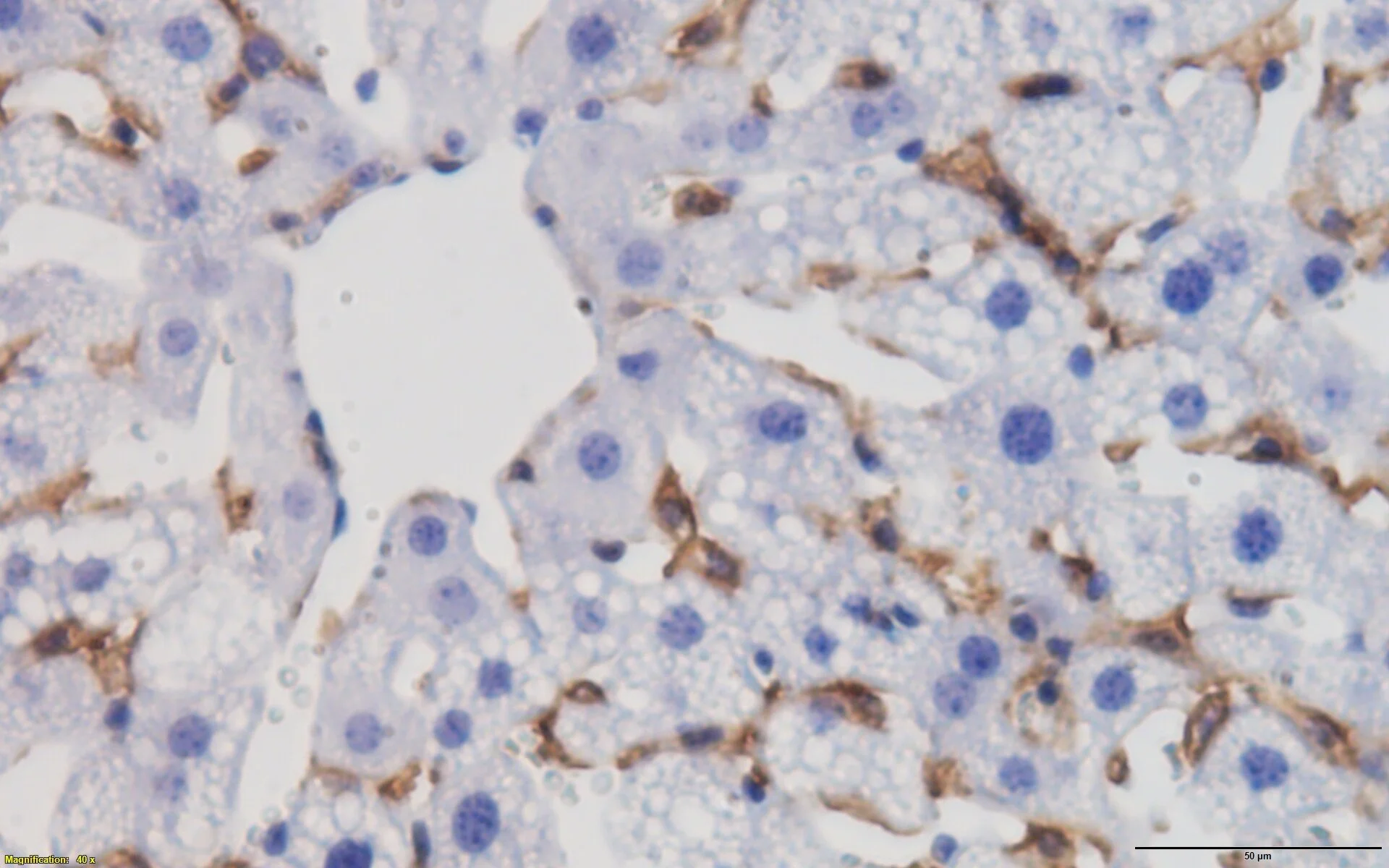
My lab has discovered the novel role of Notch, and regulators of Notch signaling, to mediate hepatic lipid metabolism. This research direction arose from the unexpected discovery that hepatocyte-specific Notch loss-of-function, or pharmacologic administration of a range of Notch inhibitors, led to a reduction in hepatic steatosis due to reduced de novo lipogenesis (Pajvani et al, Nature Medicine, 2013). To validate this, we conducted cross-sectional studies in patients undergoing liver biopsy, revealing positive correlations between hepatic Notch activity and markers of insulin resistance and hepatic triglyceride content, but independent and strongest associations with biochemical and pathologic markers of NASH (Valenti et al, Diabetes, 2013). Returning to the bench, using parallel genetic and pharmacologic approaches, we found that inhibition of hepatocyte Notch activity abrogates obesity-induced steatohepatitis and liver fibrosis, while mice with hepatocyte-specific Notch gain-of-function show exacerbated phenotypes due to increased hepatocyte secretion of the fibrogenic factor, Osteopontin (Zhu et al, Science Translational Medicine, 2018). This study also developed a novel Notch inhibitor (Nicastrin ASO) – in parallel work, we found that Nicastrin ASO reduced diet-induced dyslipidemia, due to blockade of γ-secretase complex inactivating cleavage of the LDL Receptor, leading to increased liver VLDL/LDL particle uptake (Kim et al, Cell Metabolism, 2018).
Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nature Medicine, 2013. DOI: 10.1038/nm.3259 | PubMed
Valenti L, Mendoza RM, Rametta R, Maggioni M, Kitajewski C, Shawber CJ and Pajvani UB. Hepatic Notch signaling correlates with insulin resistance and non-alcoholic fatty liver disease. Diabetes, 2013. DOI: 10.2337/db13-0769 | PubMed
Kim K, Goldberg IJ, Graham MJ, Sundaram M, Bertaggia E, Lee S, Qiang L, Haeusler RA, Metzger D, Chambon P, Yao Z, Ginsberg HN, Pajvani UB. Gamma-secretase inhibition lowers plasma triglyceride-rich lipoproteins by stabilizing the LDL receptor. Cell Metabolism, 2018. DOI: 10.1016/j.cmet.2018.02.010 | PubMed
Zhu C, Kim K, Wang X, Bartolome A, Salomao M, Dongiovanni P, Meroni M, Graham MJ, Yates KP, Diehl AM, Schwabe RF, Tabas I, Valenti L, Lavine JE and Pajvani UB. Hepatocyte Notch activation induces liver fibrosis in non-alcoholic steatohepatitis. Science Translational Medicine, 2018. DOI: 10.1126/scitranslmed.aat0344 | PubMed
Novel Modulators of de novo Lipogenesis, Liver Inflammation, and Fibrosis
We identified a novel mechanism of increased de novo lipogenesis (DNL) in obese liver, by Notch regulation of mTORC1 complex stability (Pajvani et al, Nature Medicine, 2013). Follow-up studies revealed mTORC1 regulation of mitophagy after oxidative phosphorylation uncoupling (Bartolome et al, Molecular Cell Biology, 2017) as well as the novel role of Notch-regulated, mTORC1-independent (“free”) Raptor to increase levels of the Akt phosphatase, PHLPP2, terminating post-prandial insulin action to prevent unnecessary hepatic DNL (Kim et al, Nature Communications, 2016; Kim and Pajvani, Cell Cycle, 2016). In obese mice, however, levels of the adaptor protein KCTD17 increase, leading to PHLPP2 degradation and fatty liver (Kim et al, Gastroenterology, 2017). These data identify a novel, therapeutically tractable Raptor-KCTD17-PHLPP2 axis to regulate DNL – our patent applications in this area focus on novel KCTD17 inhibitors to ameliorate obesity-induced liver disease.
Kim K, Hayden M, Qiang L, Sparling D, Purcell N, Pajvani UB. mTORC1-independent Raptor prevents hepatic steatosis by stabilizing PHLPP2. Nature Communications, 2016. DOI: 10.1038/ncomms10255 | PubMed
Kim K, Pajvani UB. “Free” Raptor - a novel regulator of metabolism. Cell Cycle, 2016. DOI: 10.1080/15384101.2016.1159835 | PubMed
Bartolome A, Garcia-Aguilar A, Ashara S-I, Kido Y, Guillen C, Pajvani UB, Benito M. MTORC1 regulates both general autophagy and mitophagy induction after oxidative phosphorylation uncoupling. Molecular Cell Biology, 2017. DOI: 10.1128/MCB.00441-17 | PubMed
Kim K, Ryu D, Dongiovanni P, Ozcan L, Nayak S, Ueberheide B, Valenti L, Auwerx J, Pajvani UB. Degradation of PHLPP2 by KCTD17, via a Glucagon-dependent pathway, promotes hepatic steatosis. Gastroenterology, 2017. DOI: 10.1053/j.gastro.2017.08.039 | PubMed